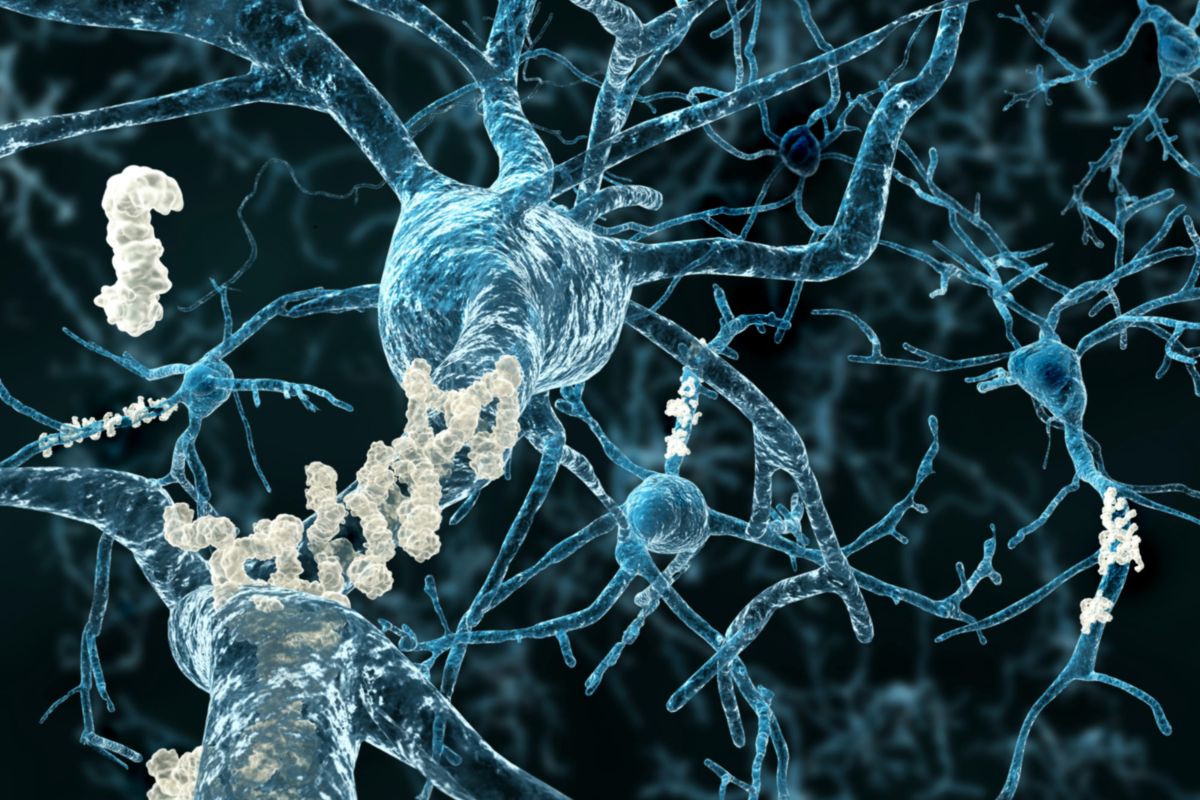
Becomes the second drug in market to slow down the dreaded memory-loss disease; Will cost $32,000 for a 12-month treatment
US Food and Drug Administration
Latest in US Food and Drug Administration
caffeine
Logan Paul, KSI’s PRIME Energy banned from supermarket shelves over high caffeine content
The decision was implemented following investigations of the beverage containing caffeine levels equivalent to nearly two Red Bulls
caffeine
Logan Paul’s PRIME Energy under US investigation over high caffeine content
PRIME Energy reportedly contains 200 milligrams of caffeine per 12 ounces, equivalent to six cans of Coca-Cola or nearly two Red Bulls
Abu Dhabi
Abu Dhabi clinical trials for Mitapivat raises hopes for thalassemia patients
Already approved by the US FDA to treat hemolytic anemia in adults with pyruvate kinase deficiency; it’s showing promise for thalassemia treatment as well
Alzheimer
Alzheimer’s disease medication Leqembi gets FDA’s Accelerated Approval
Developed by Eisai and Biogen, it slows down cognitive decline, but can also cause brain swelling and bleeding; to be priced at $26,500 for annual treatment